
Ensure safer medical devices through a systematic usability engineering process.
How to successfully apply IEC 62366-1: Practical strategies to accelerate approvals, minimize use errors, and improve patient safety.
Ensure compliance efficiently
Learn how to meet IEC 62366-1 usability engineering requirements without unnecessary delays.
Reduce use errors and risks
Apply proven methods to design safer, user-friendly medical devices that protect patients and users.
Streamline your approval process
Build a strong Usability Engineering File (UEF) and integrate usability into your risk management for faster market access.
Start strengthening your medical device usability engineering process.
Die Gebrauchstauglichkeitsuntersuchung bestimmter Medizinprodukte bedingt ein erhöhtes Maß an Realismus des Nutzungskontext, um fundierte Aussagen…
DetailsConducting usability tests with representative medical professionals and regulatory-compliant evaluation.
DetailsCarrying out analyses and evaluations as part of carcass workshops.
DetailsGenerate user-centered innovations through the use of modern UX methods.
DetailsIEC 62366-1:2015 + COR1:2016 + A1:2020
IEC TR 62366-2:2016
ISO 14971:2019 / EN ISO 14971:2019 + A11:2021
ISO 13485:2016 / EN ISO 13485:2016 + AC:2018 + A11:2021
AAMI TIR 45
Applying Human Factors and Usability Engineering to Medical Devices
Application of Human Factors Engineering Principles for Combination Products
Comparative Analyses and Related Comparative Use Human Factors Studies for a Drug-Device Combination Product Submitted in an ANDA
Contents of a Complete Submission for Threshold Analyses and Human Factors Submissions to Druf and Biologic Applications
Human Factors Studies and Related Clinical Study Considerations in Combination Product Design and Development
Medical Devices Usability Engineering Guideline (NMPA)
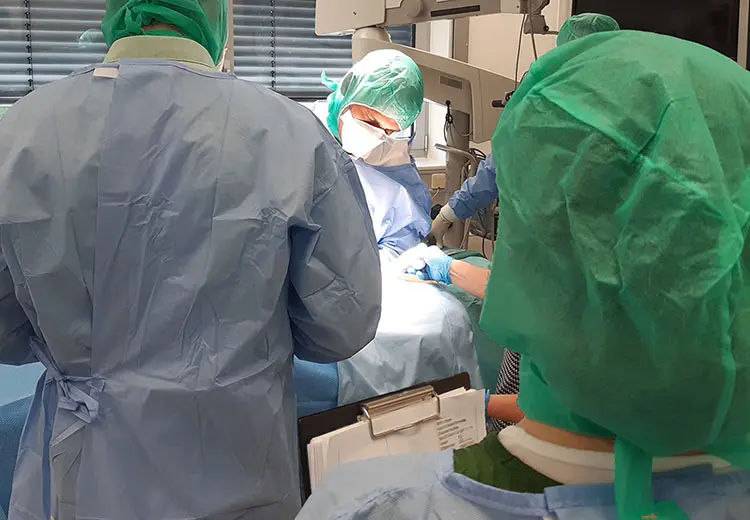
A context of use analysis, also known as User Research, marks the beginning of the user-centered development process. The goal is to thoroughly understand the future users, their workflows, and work environments. Within the requirements analysis, our experts derive relevant User Requirements & User Needs from this context of use. In accordance with normative requirements, we offer our customers the use of observing, interviewing, and testing techniques. The method spectrum includes basic techniques such as protocols, audio and video recordings, as well as the use of advanced technologies such as eye tracking & motion tracking. Through observation and interviews, we collect both qualitative and quantitative data for our customers, such as context interviews, user surveys, or focus group interviews.

Manufacturers of medical devices are required by ISO 14971 to analyze and minimize potential risks that may occur during product use. Therefore, within the scope of usability engineering, both interaction-related and usage-related risks, as well as hazardous situations, need to be considered in the development of medical devices. This leads to the creation of hazard-related use scenarios. Our experts support you in planning, conducting, and evaluating such use-related risk assessments, facilitate the integration with technical risk assessments, and provide the necessary regulatory documentation if required.
Do you want to ensure the safe use of your medical device?
Contact us to learn more.

The information collected during the analysis is systematically consolidated in the subsequent specification process. This process concludes in the regulatory-relevant use specification document and is the basis for deriving user requirements and user interface specifications. These requirement categories are then bundled and specified in the form of a functional and technical specification document. Workflow analyses make the workflow visible for the project team and help to optimize the medical device through intelligent product design. The derived task models, are transferred into regulatory-required use scenarios, that represent the basis to identify usability strengths and weaknesses of the medical device in later evaluation activities.
Wondering how to bridge the gap between qualitative research and technical specification?
Our experts will assist you in developing your requirement specification.
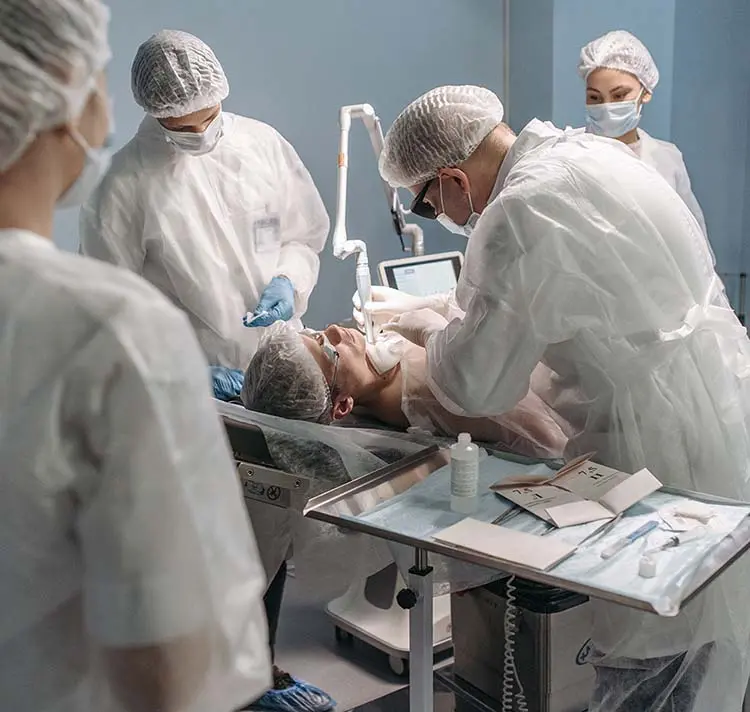
Both the FDA Guideline and IEC 62366-1 require the assurance of usability during development (formative) and at the end of development (summative), through the targeted use of usability evaluation methods. Our experts conduct expert-based inspection methods such as Cognitive Walkthroughs or Heuristic Evaluations, as well as usability tests (simulated use) with participants, or support you in conducting your own tests. The recruitment of representative test participants is an important regulatory requirement. With our pool of over 900 medical professionals, we are able to cover every medical discipline and recruit participants for tests. Standard-conforming evaluation and clear presentation of study results are also part of our expertise.
You want to test the usability of your medical devices with medical professionals before market launch?
We look forward to supporting you.
From early user interviews into the real operating room.
Discover moreInnovative yet safe and user-friendly – Mobility technology for physically disabled people.
Discover moreWe validate the suitability for use of medical devices.
Discover moreResearch - that extends into practice.
Discover more