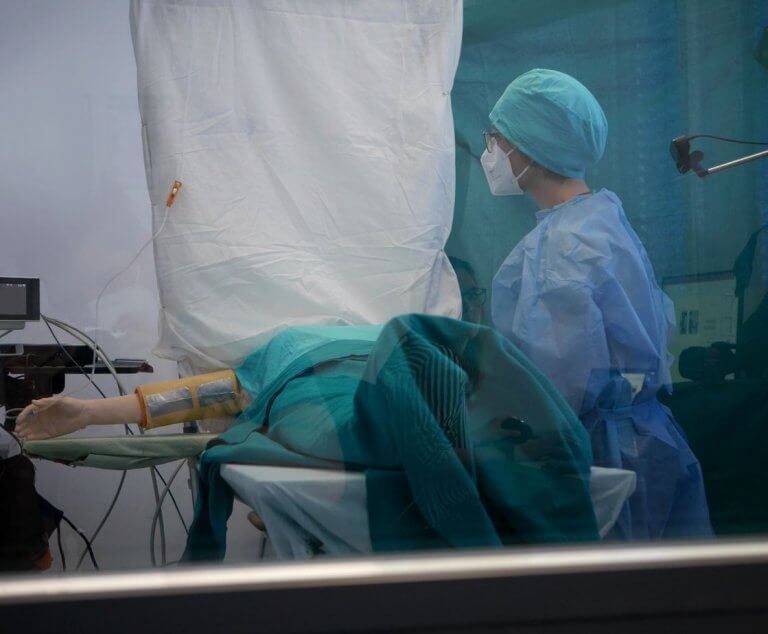
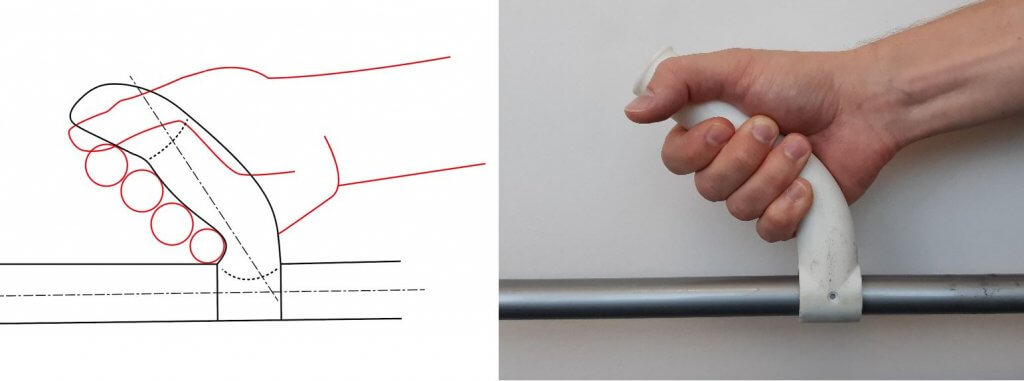
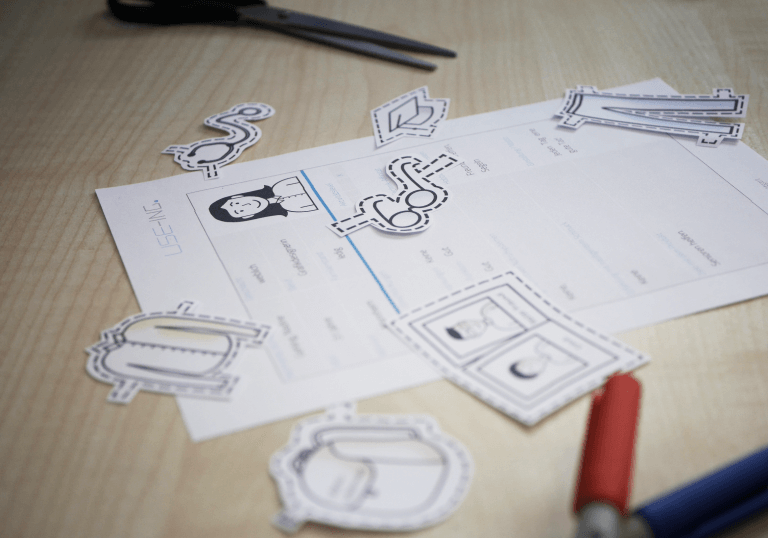
Since the introduction of the Medical Device Regulation (MDR), the examination of usability has become a focus for manufacturers of medical devices. At the heart of the so-called human factors or usability engineering process are user interface evaluations of medical products with medical professionals.
Medical progress and the innovative power of medical technology developers lead to an increasing number of interaction processes between people and technical, stationary, as well as ambulatory systems; from the perspective of the medical user as well as the treated patient. Here, the patient is usually not the user of the device, which is why medical devices typically have two interfaces to people. This interaction relationship represents the human-machine system, in which the system elements patient, doctor (or nurse), and machine are related to each other through interactions.
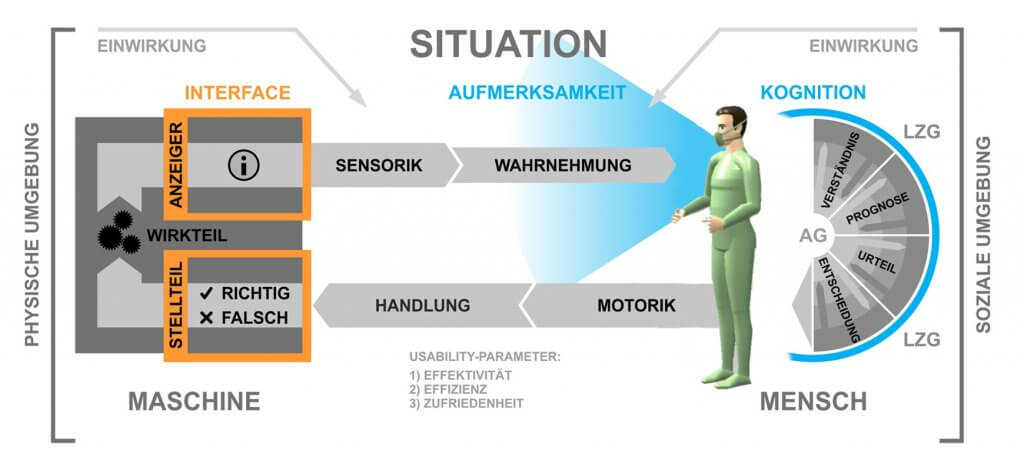
Due to the increasing use of innovative technical systems, new requirements arise from the user’s point of view in terms of safe, effective, efficient, and satisfactory operation of medical technology. The collection, implementation, and validation of these so-called usage requirements, alongside the design of user interfaces (User Interfaces) and their evaluation, are the focus of the “Usability Engineering Process”. In the development of medical devices, in addition to these components known from “User Centered Design,” the risk management of use-related risks (Use-related Risk Assessment) is an essential aspect.
Regulatory and standard requirements of the Usability Engineering Process
The establishment and implementation of a Usability Engineering Process and the documentation of all activities in a Human Factors or Usability Engineering File are part of the regulatory requirements for manufacturers of medical devices in the context of medical device approval. The normative references for usability engineering form, from a German perspective, DIN EN 62366-1:2021 and IEC/TR 62366-2:2016. The American Food and Drug Administration (FDA) raises additional regulatory requirements and presents them in its guideline document FDA-2011-D-0469 (Applying Human Factors and Usability Engineering to Medical Devices). Further requirements apply for China and the UK.
From the uss specification to user requirements engineering to use risk analysis
The specification of the application (Use Specification) represents the starting point of the Usability Engineering Process. This includes, among other things, the intended medical indication, the specification of user groups (User Profile), and the specification of all relevant usage environments. The specification of the application (Use Specification) already influences the later evaluation activities, for example, in the selection of representative test participants or test environments for the summative usability evaluation. The identification of user tasks and requirements in empirical studies of the usage context (User Research & Workflow Analyses) forms the bridge to the subsequent steps of use risk (Use-related Risk Assessment) and user requirements management (User Requirements Engineering). In the risk management process, use-related risks are analyzed and assessed based on task models. In the requirements management, the requirements necessary for the design of the human-machine interface (User Interfaces) are derived from the needs.
DID YOU KNOW?
„Risk and requirements management processes represent the collaborative processes of usability engineering. If a close interlocking is achieved here, many synergistic effects for the medical device development process can be achieved. Do you want to align your medical device development process user-centrically? Our experienced experts are happy to support you.“
User Interface Design
The subsequent phases of design and evaluation are based on risk and requirement considerations. In the User Interface Design phase, all human-product interfaces (User Interfaces) are specified within the User Interface Specification and designed using prototyping methods. Depending on the nature of the medical device to be developed, various prototyping methods can be used. For example, 3D printing and laser cutting methods are suitable. The assurance of usability (Usability) of the developed User Interface prototypes is done using targeted usability evaluation methods.
User Interface / Usability Evaluationen
Usability evaluations form the heart of the Usability Engineering Process and must be planned early on. Here, the scope, type, and timing of the evaluation methods must be adapted to the complexity of the medical device to be evaluated, not only to prove the regulatory obligation of safe and effective handling but also to enable the actual added value, the desired optimization of human-product interaction. Usability evaluation methods can be divided into inspection and user test methods.
Inspection methods are carried out by usability professionals and mostly involve checking compliance with heuristics or design guidelines. Here, experienced experts check the user interfaces of the medical device for conformity or deviation with existing and recognized guidelines. In the context of medical device development, thinking through operating procedures (Cognitive Walkthrough) is also used as an inspection method.
In contrast, the second group of methods, the user test methods, follows an empirical, user-based approach. For this purpose, it is necessary to simulate the usage context sufficiently in the usability lab and to carry out observations and interviews with users representative of the respective medical device. The gold standard test method is the Usability Test, an evaluation of usability with users based on simulated task scenarios (Test Cases), usually with participant observation.
In the Usability Engineering Process for medical devices, methods from both groups are described in the normative references. Since inspection methods involve less implementation effort, they are recommended for early phases when the user interface is not yet sufficiently concrete for a Usability Test. When which method is used must be determined in the User Interface Evaluation Plan at the beginning of the design activities. Crucial are the early planning and methodically correct implementation of the evaluations.
DID YOU KNOW?
„For usability tests, there are numerous methodological aspects that can be varied (e.g., remote-inhouse / synchronous-asynchronous), so that in principle ‘the standard usability test’ does not exist, but an individual test plan must be made depending on the specifics of the medical device to be tested. Are you facing the challenge of planning a medical usability test? Our experienced experts are happy to support you.“
If evaluations are carried out accompanying development, they are called formative evaluations. Here, the focus is on identifying optimization potentials of the human-medical product interaction and their design-technical reflection in the development process. The normative references recommend conducting several formative evaluations. Depending on the complexity, novelty, and risk profile of the user interface, two to three formative evaluations are advised.
At the end of the medical device development process, manufacturers are obliged to prove the safe and effective use of their medical device. This is done within the framework of summative usability validation or evaluation. The summative evaluation includes a normatively determined type of planning (Evaluation Plan) and documentation (Evaluation Report).
Conclusion
Medical Human Factors & Usability Engineering serves both to meet regulatory requirements and to identify market-differentiating optimization potentials. In this respect, the interlocking of usability evaluations with the application specification (Use Specification), risk management (Use-related Risk Assessment), and requirements management (User Requirements Engineering) is of the utmost importance and should be anchored interdisciplinary in the entire development team to maximize the benefits.
Do you need support in implementing your medical usability activities? Our USE-Ing. experts are ready for you.
Standards & References
- DIN EN 62366-1:2021-08 (2021): Medizinprodukte – Teil 1: Anwendung der Gebrauchstauglichkeit auf Medizinprodukte (IEC 62366-1:2015 + COR1:2016 + A1:2020)
- IEC/TR 62366-2:2016-04 (2016): Medizinprodukte Teil 2: Leitfaden zur Anwendung des Usability Engineering auf Medizinprodukte
- U.S. Department of Health and Human Services (2011): Food and Drug Administration; Center for Devices, Office of Device Evaluation (Hrsg.): Applying Human Factors and Usability Engineering to Medical Devices (FDA-2011-D-0469)
- Geis, T.; Johner, C. (2020): Usability Engineering als Erfolgsfaktor. Effizient IEC 62366- und FDA-konform dokumentieren. 2. Auflage. Beuth Verlag.
- Backhaus, C. (2010). Usability-Engineering in der Medizintechnik. Springer.
- Wiklund, M.; Kendler, J. and Strochlic, A. (2015): Usability Testing of Medical Devices. 2. Auflage, Boca Raton, Fla: CRC Press.